Kua uru a maatau hua ki te raarangi UK o nga taputapu taatai coronavirus invitro kua whakakorehia!
Ka taea e koe te tirotiro i te rarangi i runga i te paetukutuku UK Department of Health : https://www.gov.uk/.../medical-devices-regulations-2002... Ki te hiahia koe ki te hoko i a maatau hua, ka taea e koe te whakapiri mai ki a matou i i nga wa katoa!
Ripoata Whakamatau me te Tatari Silico mo StrongStep® SARS-CoV-2 Antigen Rapid Test i runga i nga momo rereke SARS-CoV-2
Kua whanakehia e SARS-CoV-2 te maha o nga huringa me nga hua kino, etahi penei i te B.1.1.7,B.1.351,B.1.2,B.1.1.28,B.1.617,Tae atu ko te riaka omicron mutant(B1.1.529) korerotia i nga ra tata nei.Hei kaihanga reagent IVD, ka aro tonu matou ki te whakawhanaketanga o nga huihuinga e tika ana, tirohia nga huringa o nga waikawa amino e tika ana me te arotake i te paanga o nga huringa ki nga reagents.
StrongStep® SARS-CoV-2 Antigen Rapid Test Whakauruhia ki te rarangi noa o te EU mo te akuaku me te haumaru kai
StrongStep® SARS-CoV-2 Antigen Rapid Test Whakauruhia ki te raarangi noa o te EU mo te akuaku me te haumaru kai, koinei tetahi o nga kaihanga iti e 100% te tairongo ina he iti iho te uara CT i te 25%.
Ko te StrongStep® SARS-CoV-2 Antigen Rapid Test kei roto i te rarangi arotake KIMI
Ko te StrongStep® SARS-CoV-2 Antigen Rapid Test i whakauruhia ki roto i te rarangi arotake KIMI.Ko te Foundation for Innovative New Diagnostics (FIND), he whakahaere e tohunga ana ki te arotake i nga mahi o nga kete i roto i te mahi rautaki me WHO.
Tauākī mō ngā wheori rerekē
I whakaatuhia e te tātaritanga raupapa raupapa ko te waahi whakarereke o te momo SARS-CoV-2 i kitea i roto i te United Kingdom, Awherika ki te Tonga me Inia kaore katoa i roto i te rohe hoahoa o te tuatahi me te tirotiro i tenei wa.StrongStep® Novel Coronavirus (SARS-CoV-2) Multiplex Waa Tuturu PCR Kit (kite mo nga ira e toru) ka taea te hipoki me te kite i nga riaka mutant (kei te tepu e whai ake nei) me te kore e pa ki te mahi i naianei.No te mea karekau he huringa o te rohe o te raupapa kitenga.
Whakarāpopotohia te Ripoata Aromatawai mai i te Whare Taapiri mo StrongStep® SARS-CoV-2 Antigen Rapid Test
We have received many certificate or EUA from different countries, such as United Kingdom, Singapore, Brazil, South Africa, Malaysia,Indonesia,Philippine, Argentine, Guatemala and so on. Also, we have send our products to many institue for evaluation, below are the summary of some data. Please contact us via guangming@limingbio.com if you need full artical of following report.
Thailand FDA COVID 19 ATK 2021 T6400429
Ina tata nei, ko te StrongStep® SARS-CoV-2 Antigen Rapid Test i hangaia e Nanjing Liming Bio-products Co. Ltd kua angitu te whiwhi tiwhikete FDA Thailand (nama rehita T 6400429,T 6400430,T 6400431,T 6400432), inaianei kua kua whakaaetia kia uru ki te maakete o Thailand.
Kua whiwhi a StrongStep® SARS-CoV-2 Antigen Rapid Test i te whakamana mahi a Paul-Ehrlich-Institut (PEI) i Tiamana!
Ina tata nei, ko te reagent detection antigen detection reagent "StrongStep® SARS-CoV-2 Antigen Rapid Test" a Nanjing LimingBio's Novel Coronavirus (SARS-CoV-2) kua whiwhi i te manatoko mahi a Paul-Ehrlich-Institut (PEI*) i Tiamana, kua riro tenei hua. kua whakamanahia e te Tiamana Federal Agency mo nga rongoa me te whakahaere taputapu rongoa (BfArM).Ko LimingBio tetahi o nga kaihanga iti i Haina kua whiwhi i te tiwhikete rua o BfArM+PEI i Tiamana.Ko te whakamatautau tere antigen a Liming Bio kua paahitia te tiwhikete whaimana a te Manatu Hauora o nga whenua maha, e tino whakaatu ana i te pai o te mahi o te kete.
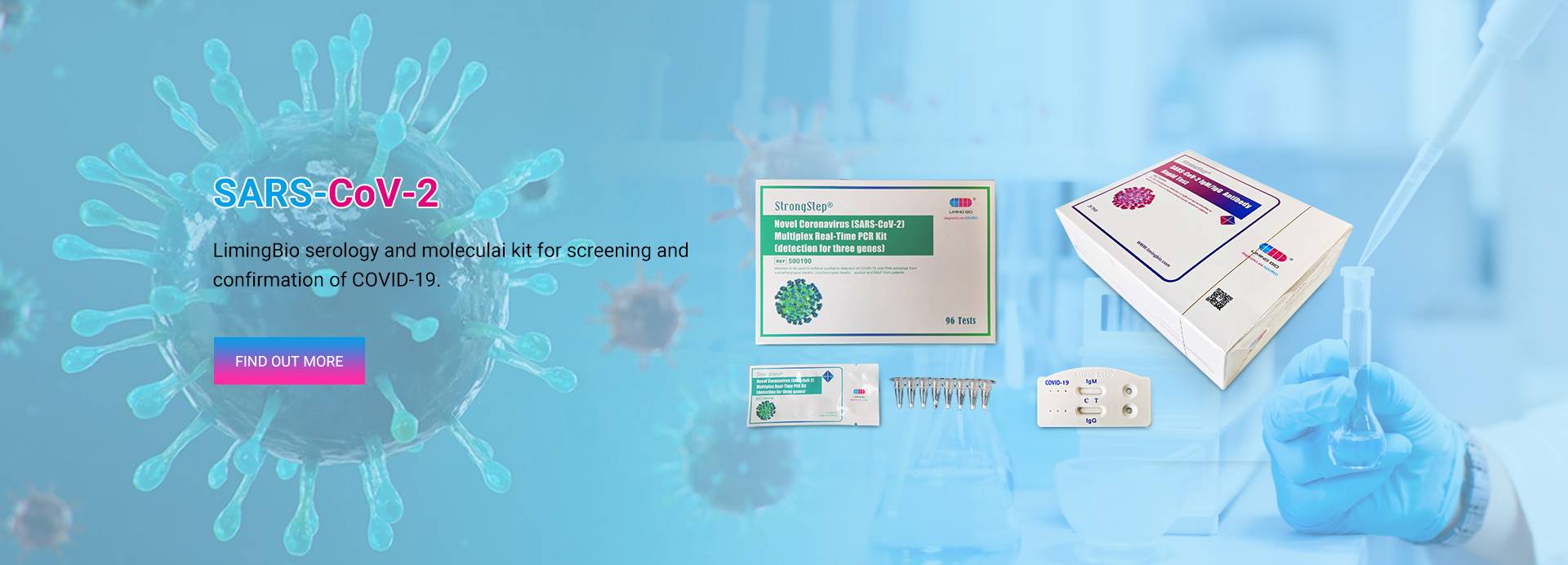
SARS-CoV-2 Antigen Rapid Test
Ko te StrongStep® SARS-CoV-2 Antigen Rapid Test he whakamatautau immunochromatographic tere mo te kitenga o te antigen COVID-19 ki te huaketo SARS-CoV-2 i roto i te korokoro o te tangata / Nasopharyngeal swab.
Novel Coronavirus (SARS-CoV-2) Multiplex Wā-tūturu Kete PCR
Ko tenei kete PCR tino tairongo, rite-ki-te whakamahi e waatea ana i roto i te whakatakotoranga lyophilized (te whakamaroke whakatio) mo te rokiroki mo te wa roa.Ka taea te kawe me te penapena i te kete ki te pāmahana rūma, ka pumau mo te tau kotahi.
O TATOU HUA HOU
MO TATOU
Nanjing Liming Bio-products Co., Ltd. i whakaturia i te tau 2001, he tohunga to taatau kamupene ki te whakawhanake, ki te hanga me te hokohoko i nga whakamatautau tere mo nga mate hopuhopu ina koa nga STD.I tua atu i te ISO13485, tata katoa o a maatau hua he tohu CE me te whakaae CFDA.He rite te ahua o a maatau hua ki te whakataurite ki etahi atu tikanga (tae atu ki te PCR, te ahurea ranei) e pau ana te waa me te utu nui.Ma te whakamahi i a maatau whakamatautau tere, ka taea e te manawanui, nga tohunga hauora ranei te whakaora i te wa roa mo te tatari na te mea me 10 meneti noa iho.



















1人份抗原卡实物图唾液版1_00_副本-300x216.png)


















